IRB review is required for REGULATED non-exempt human subjects research.
Question 1 – Is this activity regulated?
Why you are seeking review?
If you are conducting regulated non-exempt human subject research, you have no choice; either IRB review or an exemption is required. Investigators of non-regulated studies often seek IRB review for other voluntary and involuntary reasons such as for journal publication, institutional policy and personal ethical principles.
Note: Investigators are sometimes asked to “get IRB review” when, in fact, it may be more appropriate to obtain an exemption or a determination that there is no human subject research. (If this happens to you and the person insists only approval will do, please call. Sometimes logic works and sometimes it is better to proceed with IRB review than fight.)
What are the sources of regulation?
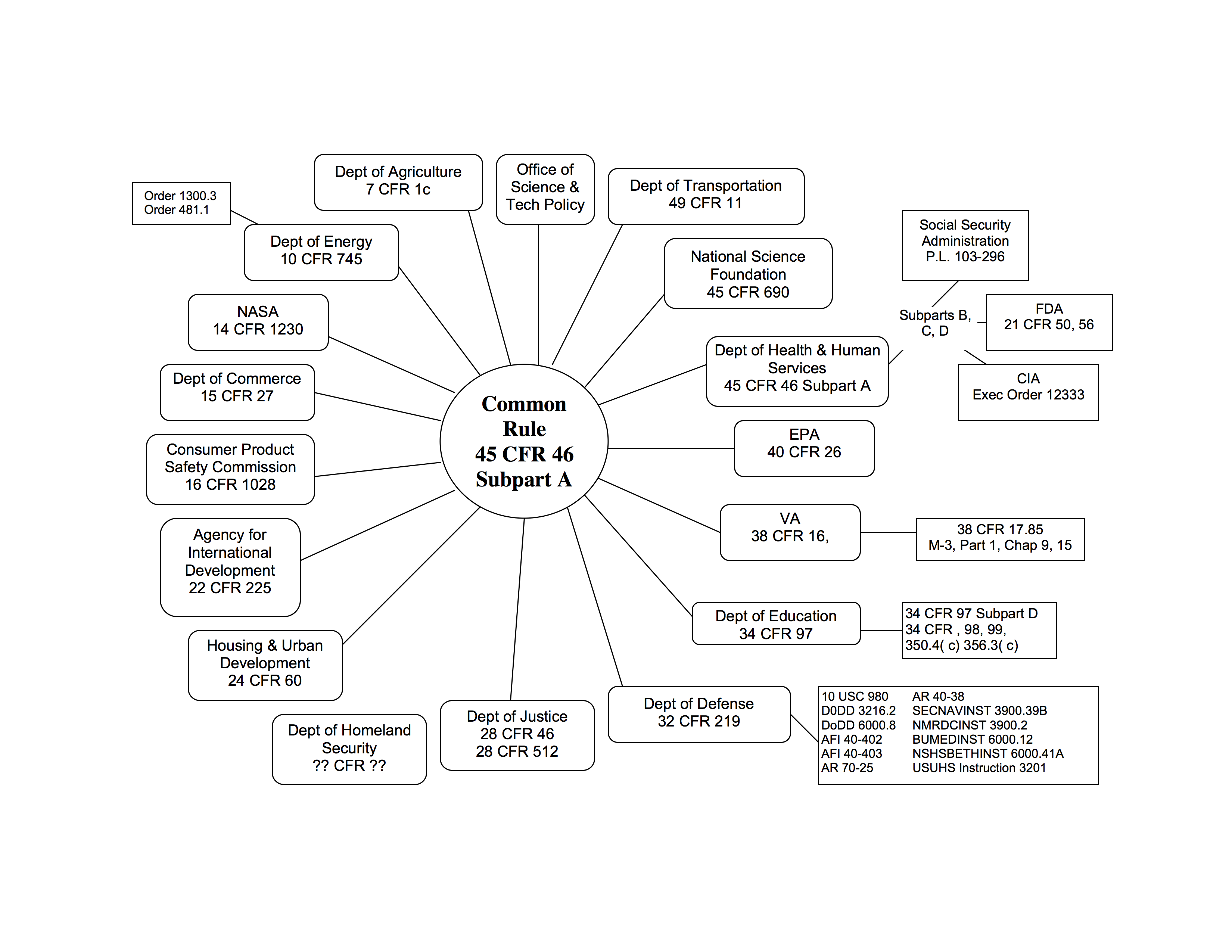
- Human Subject Research that is funded by a grant from almost any federal agency is subject to regulation. The basic rule adopted by most federal agencies is called The Common Rule. It is found at Title 45, Code of Federal Regulations, Part 46, subpart A.
- Many institutions have policies that require IRB review regardless of funding. Most have adopted the Common Rule unless they are doing work on behalf of FDA-regulated sponsors.
- Many journals require evidence of IRB review.
- Food and Drug Administration (FDA) regulations might apply even to minimal risk studies. If so, some most exemptions and waivers of consent will not be available.
When is review voluntary?
- Some professional codes require independent review of studies. Ethical codes are not regulation but do lead to good behavior.
- Some independent investigators simply want the comfort of knowing that additional eyes looked at the study.
Please be aware the voluntary reviews are treated the same way as required reviews and investigators are expected to adhere to the same standards.
CONTINUE IF your project is regulated or if you wish voluntary review.
REGULATIONS:
The Common Rule Graphic illustrates the agencies that have adopted it and gives the citation.
For purposes of the decision tree, we are using the regulations found at Title 45 of the Code of Federal Regulations, Part 46.102 http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html
