Q: When is IRB review required?
A: IRB review is required for regulated, non-exempt, human subjects research.
Every word in this answer has been the subject of IRB conference workshops.
SBER studies have many threshold decisions unlike clinical research that almost always require review by the full convened IRB. Selecting too low a level can result in extra corrective work but selecting too high a level can yield questions more appropriate for other studies. Researchers in the SBER realm must be conversant with the decision tree.
There are many versions of the decision trees. Here are three:
- The official decision tree from OHRP (Office for Human Research Protections, DHHS). http://www.hhs.gov/ohrp/policy/checklists/decisioncharts.html
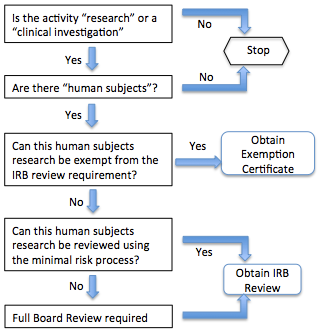
- A very simplified decision tree (see below)
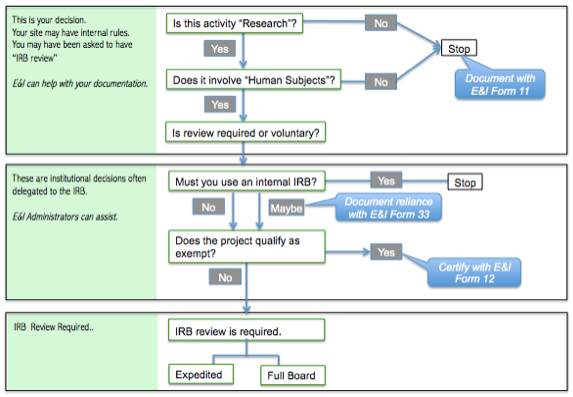
- An E&I decision tree mentioning our forms (see below)
You should be able to answer these questions after reviewing this section:
- When is IRB review required?
- What is “Research”?
- What defines a “Human Subject”?
- What can be exempt from IRB review?
CAUTION and NOTICE
- Risk is not an issue. Risk is not involved in the threshold decisions.
- Consent is almost always required but there is much more flexibility in how it occurs. Think about consent as common courtesy.
- Consent documentation may not be required – but is not a bad idea either – until the IRB review stage is reached. Think about documentation as ranging from the traditional full signed document to handing out an information sheet and asking for an action in response to you documenting the discussion with a checkbox in your notes. They all have a place.
DECISION TREE 1: This first decision tree sets the stage. It is the easiest but lacks any explanation.

DECISION TREE 2: This decision tree is referencing our forms. (By institutional we mean your company or organization.)